Hope Biosciences Research Foundation
A Greater Houston Community partnership growing one stem cell at a time

What do Greater Houston Community Foundation and an innovative adult cell therapy nonprofit have in common? Both care a lot about Houston and her people, and both work daily to meet the needs of their shared community. Both organizations also value how they do business, as much as what they do. What more could Houston ask for?
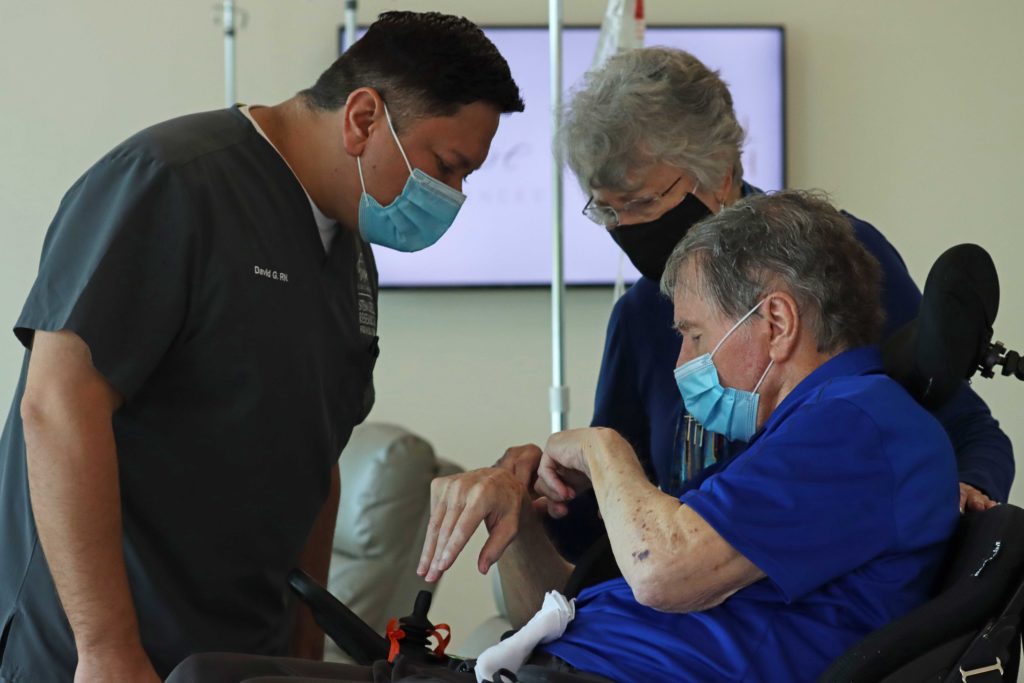
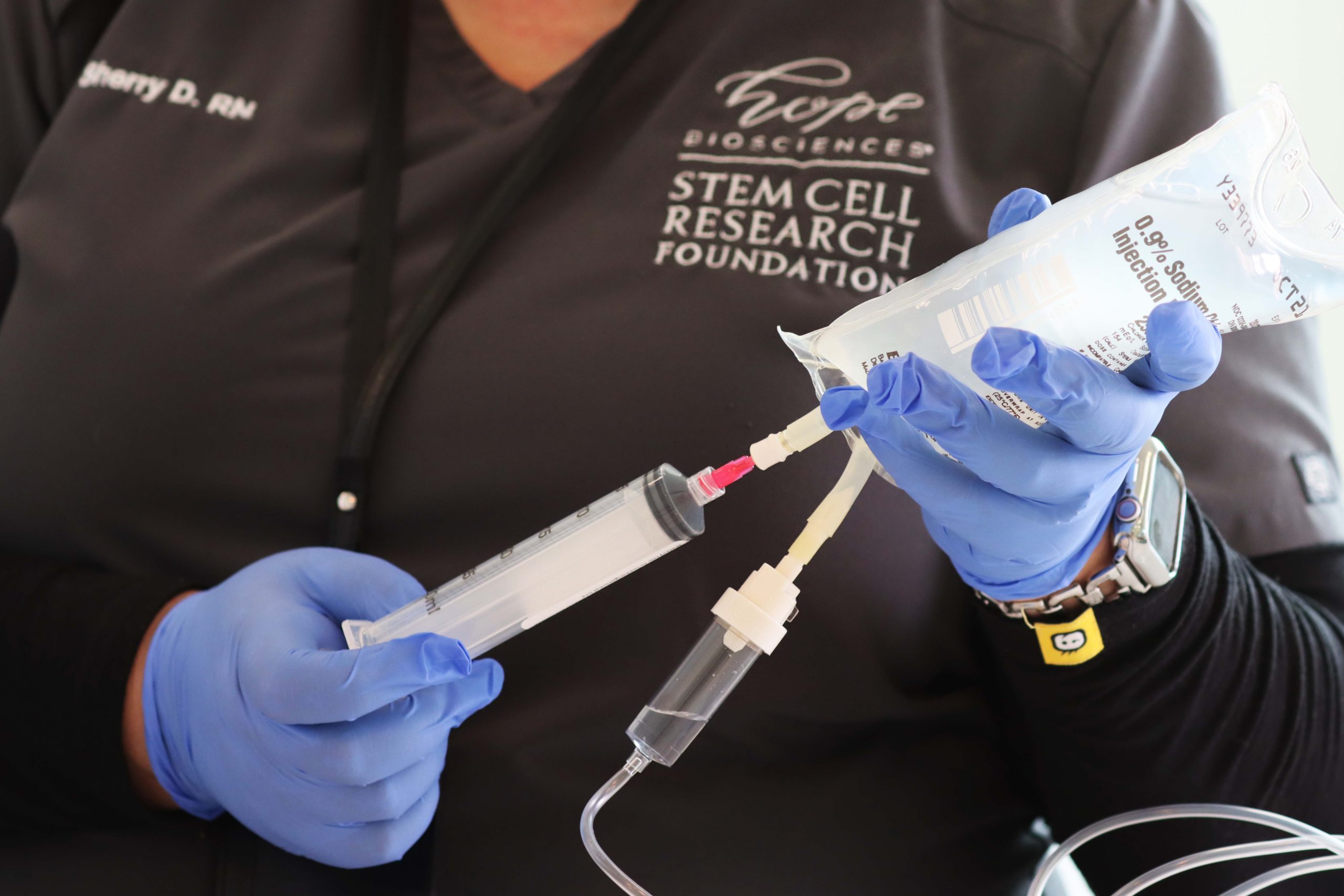
Hope Biosciences Research Foundation (HBRF), headquartered in Sugar Land, Texas (west Houston), exists to revolutionize medicine by accelerating translational research in regenerative medicine to develop cures for all. Founded in 2020 only 20 minutes from Houston’s Medical Center, HBRF conducts FDA-authorized research in adult cell therapy, pushing to get biotechnologies to patients suffering from chronic degenerative diseases and injuries deemed “incurable” by conventional medicine. To date, HBRF has obtained FDA authorization for more than 30 clinical studies. Clinical trial authorizations encompass COVID-19 prevention and treatment, “Long Haul” COVID, Parkinson’s Disease, and Multiple Sclerosis (MS). Expanded access protocol authorizations include nervous system conditions such as amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS), Cerebral Palsy, spinal cord injury, polyneuropathy, and muscular dystrophy; as well as immune conditions such as lupus; and chronic musculoskeletal pain, severe osteoarthritis, and psoriatic arthritis.
A simple list of conditions studies is insufficient to capture many exciting facets of how HBRF operates. HBRF regularly receives media attention and support from Houston area grant-making bodies for ongoing work in COVID-19, including “Long Haul” COVID, serving 90 patients across two FDA-authorized clinical trials in the condition. So-called “long haulers” are patients who continue to display COVID symptoms anywhere from three to more than twelve weeks after recovery from the virus, including fatigue, shortness of breath, cough, headache, body aches, and loss of taste and smell, among others. HBRF’s Parkinson’s Disease program also continues to grow thanks to significant local support. Work in Parkinson’s now encompasses five studies, including a study for patients aged 76 years and older, the only FDA-authorized study in this age range in the world. In 2022, HBRF launched a first-of-kind Patient Registry, an online portal that allows patients to pre-screen for FDA-authorized studies and be placed on a waitlist, as well as helping HBRF identify areas of most pressing unmet community needs.
As a leading philanthropic grant maker in the Houston area, Greater Houston Community Foundation partners with donors to create a meaningful and long-lasting impact in our shared community. We are proud to support HBRF through the administration of its foundation. Healthcare affects us all, and right here, real work is taking place to make advanced biotechnologies available to people in need. The future is bright in Houston.